| Structure | Name/CAS No. | Articles |
|---|---|---|
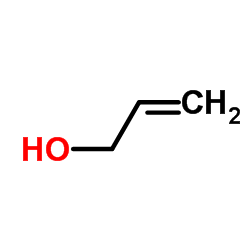 |
Allyl alcohol
CAS:107-18-6 |
|
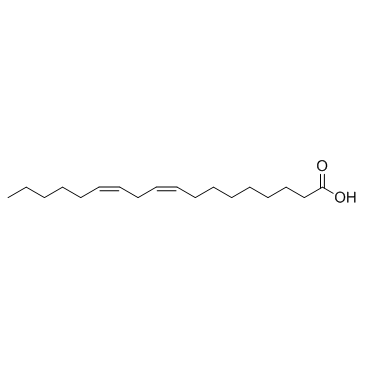 |
Linoleic acid
CAS:60-33-3 |
|
 |
Sodium (9Z,12Z)-octadeca-9,12-dienoate
CAS:822-17-3 |
|
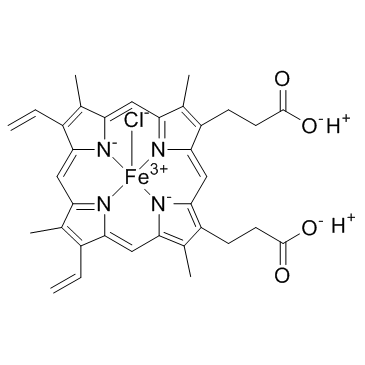 |
Hemin
CAS:16009-13-5 |