| Structure | Name/CAS No. | Articles |
|---|---|---|
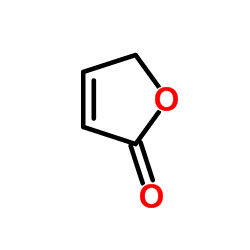 |
2(5H)-Furanone
CAS:497-23-4 |
|
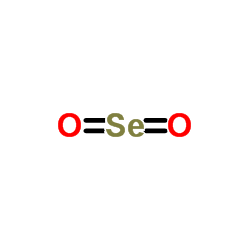 |
Selenium dioxide
CAS:7446-08-4 |