Synthesis and biological activity of desmethoxy analogues of coruscanone A.
Lucie Tichotová, Eliška Matoušová, Marcel Spulák, Jiří Kuneš, Ivan Votruba, Vladimír Buchta, Milan Pour
Index: Bioorg. Med. Chem. Lett. 21(20) , 6062-6, (2011)
Full Text: HTML
Abstract
A series of simple desmethoxy analogues of coruscanone A was prepared via a novel version of Ti(iPrO)(4)-mediated Knoevenagel condensation of cyclopentenedione with substituted benzaldehydes and cinnamic aldehydes, and the compounds were evaluated for antifungal activity and cytotoxicity. The most potent 2-benzylidenecyclopent-4-ene-1,3-dione possessed antifungal effect comparable to coruscanone A and a somewhat broader spectrum of activity against Candida species. The compound was also superior to fluconazole against several non-albicans Candida sp. Evaluation of the ability of the compound to influence cell proliferation using two different assays showed that 2-benzylidenecyclopent-4-ene-1,3-dione has lower cytotoxicity compared to the natural product.Copyright © 2011 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
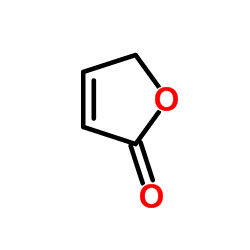 |
2(5H)-Furanone
CAS:497-23-4 |
C4H4O2 |
|
Comparative safety of the antifouling compound butenolide an...
2014-04-01 [Aquat. Toxicol. 149 , 116-25, (2014)] |
|
Proteomic changes in brain tissues of marine medaka (Oryzias...
2014-12-01 [Aquat. Toxicol. 157 , 47-56, (2014)] |
|
Alpha, beta-unsaturated lactones 2-furanone and 2-pyrone ind...
2013-09-12 [Toxicol. Lett. 222(1) , 64-71, (2013)] |
|
The butenolide signaling molecules SRB1 and SRB2 induce lank...
2012-07-09 [ChemBioChem. 13(10) , 1447-57, (2012)] |
|
Acute toxicity of the antifouling compound butenolide in non...
2011-01-01 [PLoS ONE 6(8) , e23803, (2011)] |