Journal of Organic Chemistry
2012-07-20
A protocol for an asymmetric synthesis of γ-amino acids.
Leleti Rajender Reddy, Kapa Prasad, Mahavir Prashad
Index: J. Org. Chem. 77(14) , 6296-301, (2012)
Full Text: HTML
Abstract
A new and practical method for the asymmetric synthesis of γ-amino acids from β,γ-butenolides by an in situ esterification, condensation, and reduction in a one-pot procedure is described. This method is quite general for the preparation of both enantiomers of aryl or aliphatic γ-amino acids in high yields. These γ-amino-acid derivatives were also shown to be versatile synthetic intermediates for further transformations by their conversion to γ-lactams, δ-amino alcohols, and hydrolysis products in high yields with no racemization.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
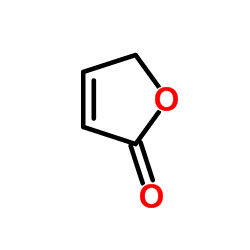 |
2(5H)-Furanone
CAS:497-23-4 |
C4H4O2 |
Related Articles:
More...
|
Comparative safety of the antifouling compound butenolide an...
2014-04-01 [Aquat. Toxicol. 149 , 116-25, (2014)] |
|
Proteomic changes in brain tissues of marine medaka (Oryzias...
2014-12-01 [Aquat. Toxicol. 157 , 47-56, (2014)] |
|
Alpha, beta-unsaturated lactones 2-furanone and 2-pyrone ind...
2013-09-12 [Toxicol. Lett. 222(1) , 64-71, (2013)] |
|
The butenolide signaling molecules SRB1 and SRB2 induce lank...
2012-07-09 [ChemBioChem. 13(10) , 1447-57, (2012)] |
|
Acute toxicity of the antifouling compound butenolide in non...
2011-01-01 [PLoS ONE 6(8) , e23803, (2011)] |