| Structure | Name/CAS No. | Articles |
|---|---|---|
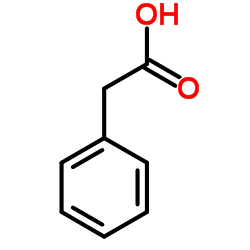 |
Phenylacetic acid
CAS:103-82-2 |
|
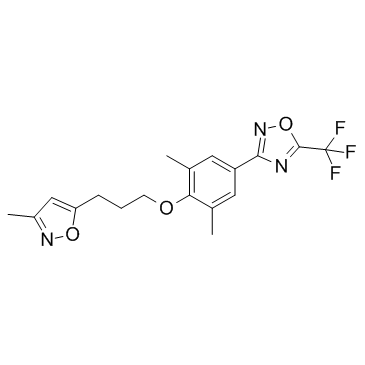 |
Pleconaril
CAS:153168-05-9 |