|
~96% |
|
~86% |
|
~85% |
|
~98% |
|
~97% |
|
~81% |
|
~94% |
|
~88% |

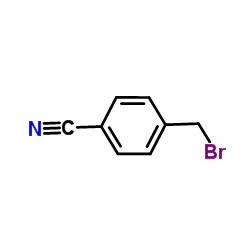
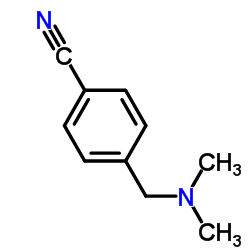
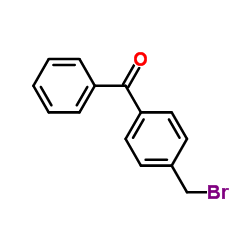
![[4-[(dimethylamino)methyl]phenyl]-phenylmethanone Structure](https://image.chemsrc.com/caspic/346/13991-01-0.png)
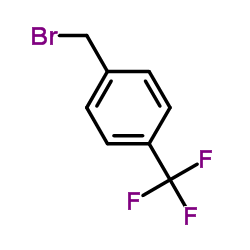
![N,N-dimethyl-1-[4-(trifluoromethyl)phenyl]methanamine Structure](https://image.chemsrc.com/caspic/408/71740-33-5.png)
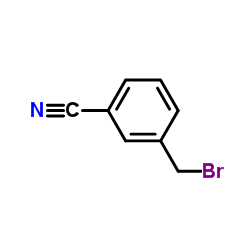
![3-[(dimethylamino)methyl]benzonitrile Structure](https://image.chemsrc.com/caspic/302/42967-27-1.png)
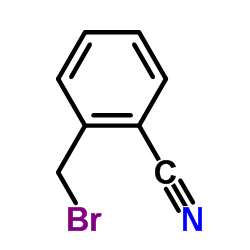
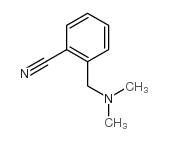
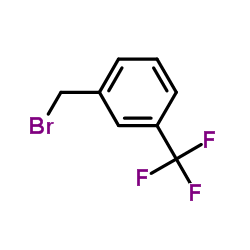
![N,N-dimethyl-1-[3-(trifluoromethyl)phenyl]methanamine Structure](https://image.chemsrc.com/caspic/222/713-93-9.png)
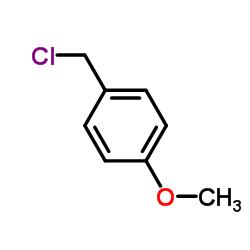
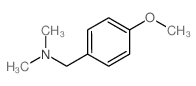
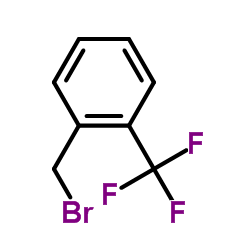
![N,N-Dimethyl-1-[2-(trifluoromethyl)phenyl]methanamine Structure](https://image.chemsrc.com/caspic/151/712-20-9.png)