|
~% |
|
~% |
|
~% |
|
~99% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~80% |

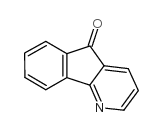
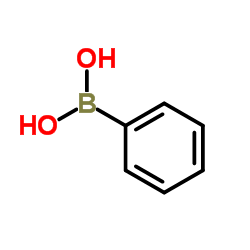
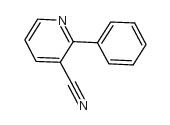
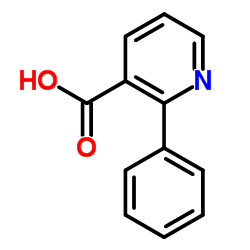
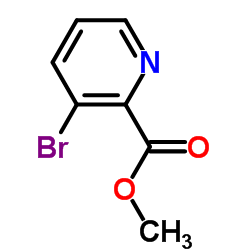
![indeno[2,1-b]pyridin-9-one Structure](https://image.chemsrc.com/caspic/495/57955-12-1.png)
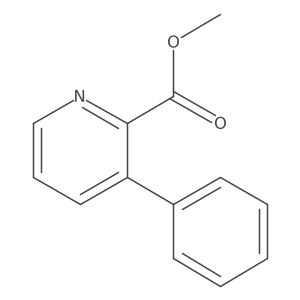
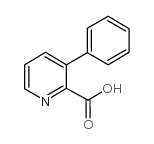
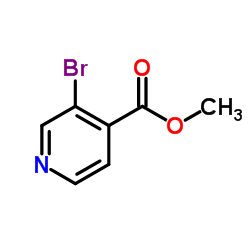
![5H-Indeno[1,2-c]pyridin-5-one Structure](https://image.chemsrc.com/caspic/469/18631-22-6.png)
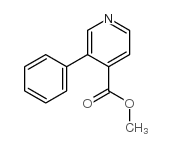

![methyl 5-hydroxyindeno[1,2-b]pyridine-5-carboxylate Structure](https://image.chemsrc.com/caspic/137/97677-73-1.png)
![5-((trimethylsilyl)oxy)-5H-indeno[1,2-b]pyridine-5-carbonitrile Structure](https://image.chemsrc.com/caspic/179/439118-19-1.png)
