| Structure | Name/CAS No. | Articles |
|---|---|---|
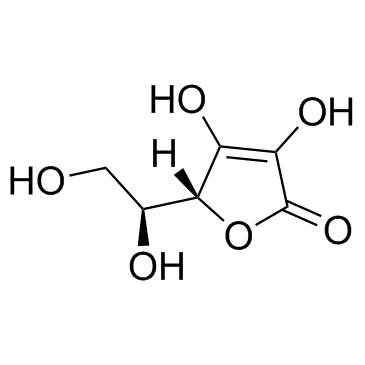 |
Ascorbic acid
CAS:50-81-7 |
|
 |
Syringic acid
CAS:530-57-4 |
|
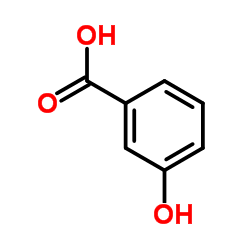 |
3-Hydroxybenzoicacid
CAS:99-06-9 |
|
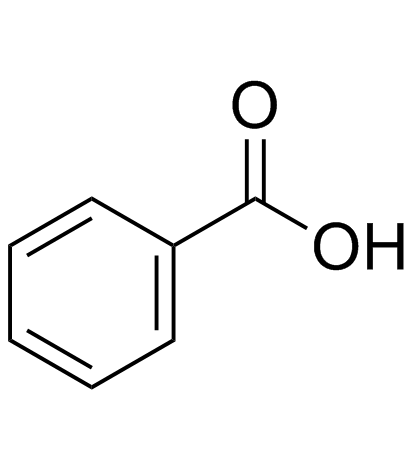 |
benzoic acid
CAS:65-85-0 |
|
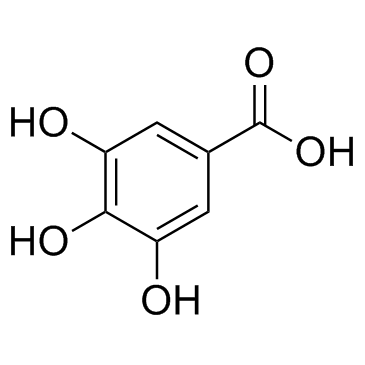 |
Gallic acid
CAS:149-91-7 |
|
 |
Hippuric acid
CAS:495-69-2 |
|
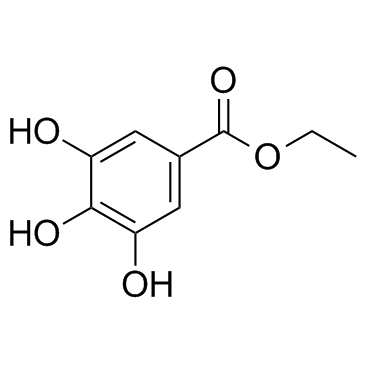 |
Ethyl gallate
CAS:831-61-8 |