| Structure | Name/CAS No. | Articles |
|---|---|---|
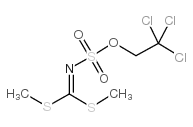 |
S,S-DIMETHYL N-(2,2,2-TRICHLOROETHOXYSULFONYL)-CARBONIMIDODITHIONATE
CAS:882739-46-0 |
|
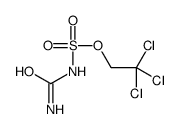 |
Aminocarbonylsulfamic acid,3,3,3-trichloroethoxy ester,Tces-Urea
CAS:882739-31-3 |