| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Potassium
CAS:7440-09-7 |
|
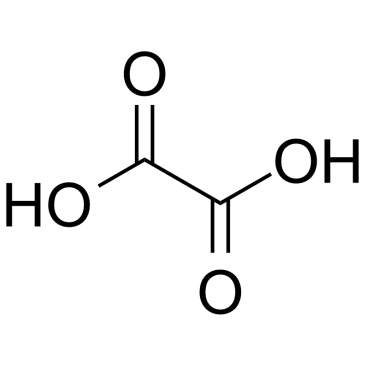 |
Oxalic acid
CAS:144-62-7 |
|
 |
potassium hydride
CAS:7693-26-7 |
|
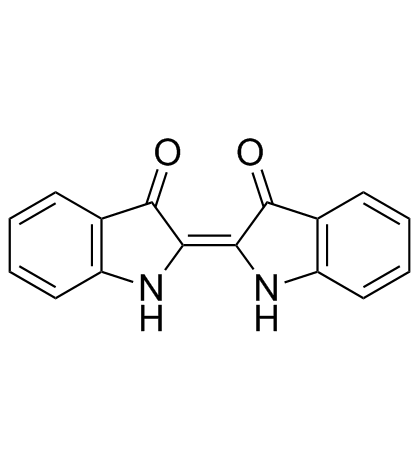 |
Indigo
CAS:482-89-3 |