Nature Structural & Molecular Biology
2014-12-01
Structure of AMP-PNP-bound BtuCD and mechanism of ATP-powered vitamin B12 transport by BtuCD-F.
Vladimir M Korkhov, Samantha A Mireku, Dmitry B Veprintsev, Kaspar P Locher
Index: Nat. Struct. Mol. Biol. 21(12) , 1097-9, (2014)
Full Text: HTML
Abstract
The reaction mechanism of BtuCD-F-catalyzed vitamin B12 transport into Escherichia coli is currently unclear. Here we present the structure of the last missing state in the form of AMP-PNP-bound BtuCD, trapped by a disulfide cross-link. Our structural and biochemical data allow a consistent mechanism to be formulated, thus rationalizing the roles of substrate, ATP and substrate-binding protein.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
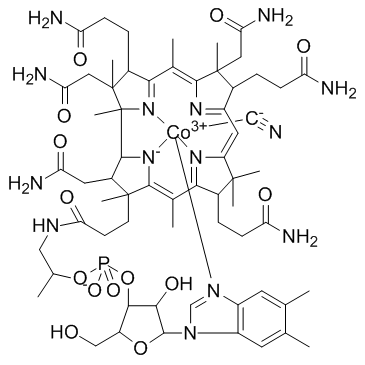 |
Vitamin B12
CAS:68-19-9 |
C63H88CoN14O14P |
Related Articles:
More...
|
Alanine Scanning Mutagenesis Identifies an Asparagine–Argini...
2008-01-01 [J. Mol. Biol. 426(12) , 2328-45, (2014)] |
|
Tissue vitamin concentrations are maintained constant by cha...
2014-01-01 [Biosci. Biotechnol. Biochem. 78(12) , 2102-9, (2014)] |
|
Effect of concentrate feeder design on performance, eating a...
2015-06-01 [J. Anim. Sci. 93 , 3018-33, (2015)] |
|
Solubilization of gliadins for use as a source of nitrogen i...
2015-02-01 [Food Chem. 168 , 439-44, (2014)] |
|
Disease mutations in desmoplakin inhibit Cx43 membrane targe...
2014-09-15 [J. Cell Biol. 206(6) , 779-97, (2014)] |