| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Zirconium dioxide
CAS:1314-23-4 |
|
 |
zirconium
CAS:7440-67-7 |
|
 |
Lead
CAS:7439-92-1 |
|
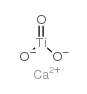 |
calcium titanate
CAS:12049-50-2 |