| Structure | Name/CAS No. | Articles |
|---|---|---|
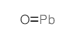 |
Lead monoxide
CAS:1317-36-8 |
|
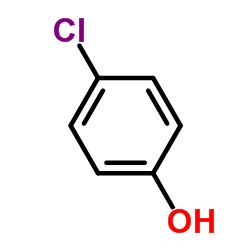 |
4-Chlorophenol
CAS:106-48-9 |