Chemical & Pharmaceutical Bulletin
2009-02-01
Deprotection of the indole (N(ind))-formyl (For) group on tryptophan employing a new reagent, N,N'-dimethylethylendiamine (DMEDA) in an aqueous solution.
Takenao Odagami, Yuko Tsuda, Yuji Kogami, Hiroyuki Kouji, Yoshio Okada
Index: Chem. Pharm. Bull. 57(2) , 211-3, (2009)
Full Text: HTML
Abstract
The deprotection of the indole (N(ind))-formyl (For) group on Trp was achieved in a 95% yield using N,N'-dimethylethylendiamine (DMEDA) (1.5, 2.0, 3.0 eq) in water at room temperature. A new reagent was successfully applied to the deprotection of a model peptide, H-Phe-Trp(N(ind)-For)-Lys-Tyr-OH, to give H-Phe-Trp-Lys-Tyr-OH in a 91% yield.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
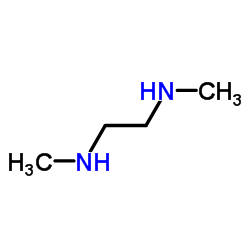 |
1,2-Dimethylethylenediamine
CAS:110-70-3 |
C4H12N2 |
Related Articles:
More...
|
Redox effects and cytotoxic profiles of MJ25 and auranofin t...
2015-06-30 [Oncotarget 6 , 16488-506, (2015)] |
|
EPR spectra of Cu(2+) doped [Zn(sac)2(dmen)] and [Zn(sac)2(p...
2007-10-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 68(2) , 394-8, (2007)] |
|
Thermodynamics of the interaction of Pd(dmen)(H₂O)₂²⁺ with b...
2012-06-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 91 , 383-8, (2012)] |
|
Parallel synthesis of a library of benzoxazoles and benzothi...
2006-03-03 [J. Org. Chem. 71(5) , 1802-8, (2006)] |
|
Transition-metal-free intramolecular N-arylations.
2012-04-06 [Org. Lett. 14(7) , 1892-5, (2012)] |