| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
L-Nicotine
CAS:54-11-5 |
|
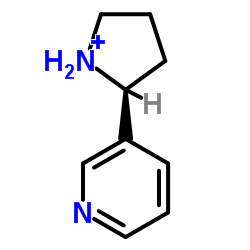 |
(±)-Nornicotine
CAS:5746-86-1 |