Novel cyclization chemistry especially suited for biologically derived, unprotected peptides.
S J Wood, R Wetzel
Index: Int. J. Pept. Protein Res. 39(6) , 533-9, (1992)
Full Text: HTML
Abstract
A novel method is described for the cyclization of peptides--or segments of polypeptides--which requires a free N-terminal alpha-amino group and a distal amino acid residue containing a nucleophilic side chain. The reaction is conducted in two steps, both in the aqueous phase. The first step involves acylation of the N-terminal alpha-amino group with iodoacetic anhydride at pH 6. This acylation reaction has greater than 90% specificity for peptide alpha-amino groups and gives no alkylation of Arg, His, Lys or Met by the iodoacetate side product (R. Wetzel et al., Bioconjugate Chem., 1, 114-122, 1990). In the second step, the acylation reaction mixture or the isolated iodoacetyl-peptide is incubated at room temperature to give the cyclic peptide formed by reaction of the nucleophilic side chain with the iodoacetyl moiety. The pH dependence of the cyclization reaction by Met, Lys, Arg or His is consistent with the pKa of the nucleophilic side chain. Thus, peptides containing Met plus other nucleophilic amino acids should preferentially cyclize via Met at low pH. In this paper, preparation of cyclic peptides containing 3-6 amino acids is described; the full range of ring sizes and sequences which can undergo this cyclization has not been further explored. Preliminary results suggest that this method is also fairly general with respect to the amino acid sequence being cyclized. The reaction appears to be particularly suited for cyclization via Lys and Met side chains. All of the cyclized products are sufficiently stable for many biological applications.(ABSTRACT TRUNCATED AT 250 WORDS)
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
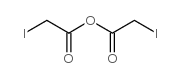 |
iodoacetic anhydride
CAS:54907-61-8 |
C4H4I2O3 |
|
Synthesis and evaluation of nuclear targeting peptide-antise...
1995-01-01 [Bioconjug. Chem. 6(1) , 101-8, (1995)] |
|
A novel method for the incorporation of glycoprotein-derived...
1992-01-01 [Bioconjug. Chem. 3(5) , 391-6, (1992)] |
|
Peptide affinity chromatography media that bind N(pro) fusio...
2010-10-01 [J. Chromatogr. A. 1217(40) , 6203-13, (2010)] |
|
A general method for highly selective cross-linking of unpro...
1990-01-01 [Bioconjug. Chem. 1(2) , 114-22, (1990)] |