| Structure | Name/CAS No. | Articles |
|---|---|---|
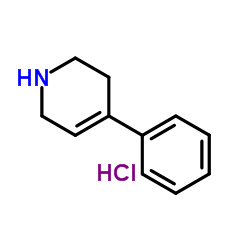 |
4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride
CAS:43064-12-6 |
|
 |
4-Phenylpyridine
CAS:939-23-1 |