| Structure | Name/CAS No. | Articles |
|---|---|---|
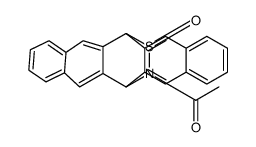 |
13,6-N-SULFINYLACETAMIDOPENTACEN
CAS:454675-76-4 |
|
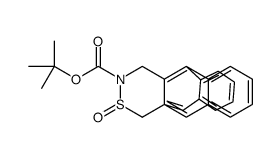 |
13,6-(EPITHIOIMINO)PENTACENE-16-CARBOXYLIC ACID, 6,13-DIHYDRO-, TERT BUTYL ESTER, 15-OXIDE
CAS:794586-44-0 |