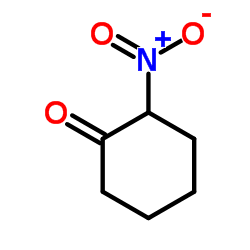
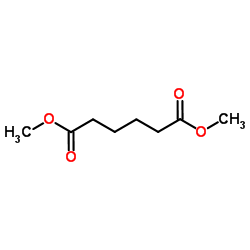
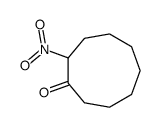
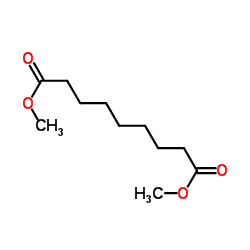
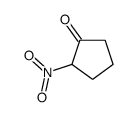
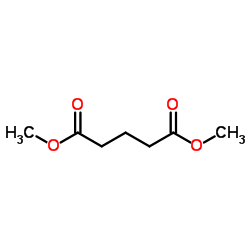
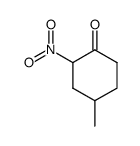
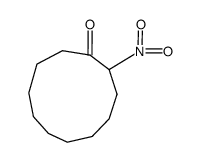
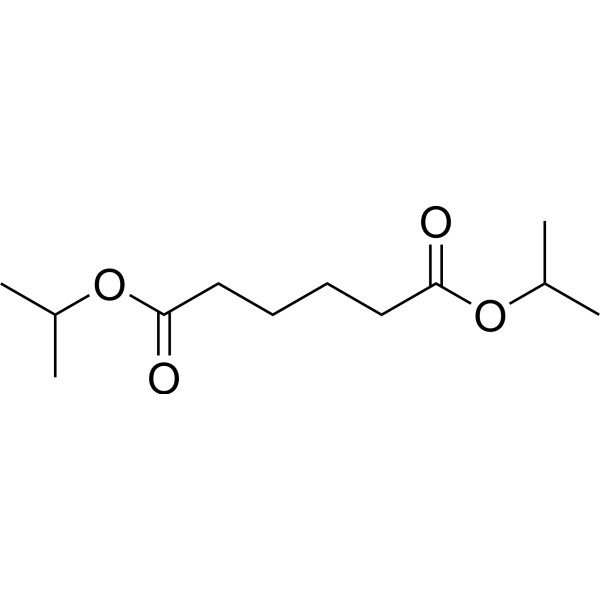
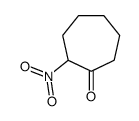
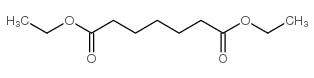
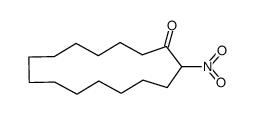
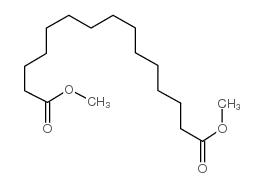
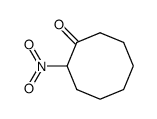
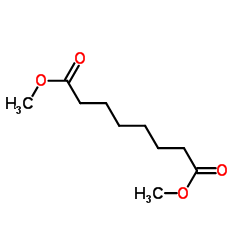
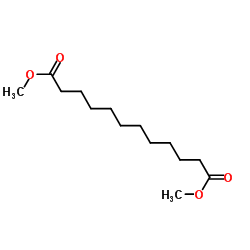
|
~94% |
|
~78% |
|
~89% |
|
~76% |
|
~83% |
|
~71% |
|
~84% |
|
~80% |
|
~88% |
|
~86% |