| Structure | Name/CAS No. | Articles |
|---|---|---|
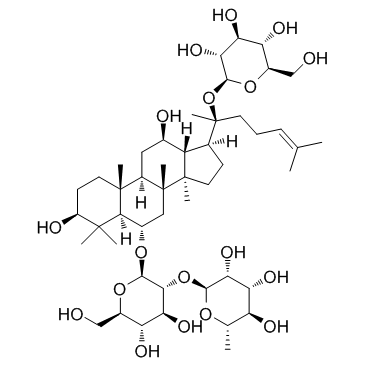 |
Ginsenoside Re
CAS:52286-59-6 |
|
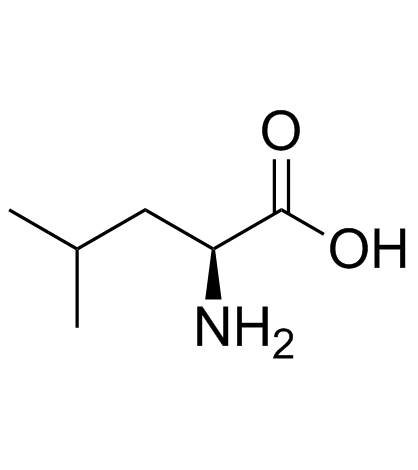 |
L-leucine
CAS:61-90-5 |