Chemosphere
2011-01-01
Kinetics and mechanism of propachlor reductive transformation through nucleophilic substitution by dithionite
C.S. Liu, K. Shih, L. Wei, F. Wang, F.B. Li, C.S. Liu, K. Shih, L. Wei, F. Wang, F.B. Li, C.S. Liu, K. Shih, L. Wei, F. Wang, F.B. Li
Index: Chemosphere 85(9) , 1438-43, (2011)
Full Text: HTML
Abstract
Highlights ► The reductive dechlorination of propachlor was efficiently achieved by dithionite. ► The transformation of propachlor initiated by dithionite follows second-order kinetics. ► Dechlorination was found to be the first and necessary step of propachlor transformation initiated by dithionite. ► Propachlor nucleophilic substitution by dithionite was confirmed as the S N2 mechanism.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
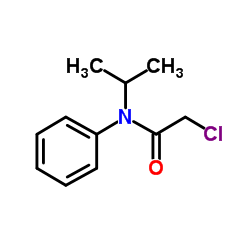 |
Propachlor
CAS:1918-16-7 |
C11H14ClNO |
Related Articles:
More...
|
Glutathione-dependent cytotoxicity of the chloroacetanilide ...
1999-01-01 [Cell Biol. Toxicol. 15(5) , 325-32, (1999)] |
|
Characterization of glutathione conjugates of chloroacetanil...
2007-01-01 [Rapid Commun. Mass Spectrom. 21(24) , 4017-22, (2007)] |
|
Correlation analyses for bimolecular nucleophilic substituti...
2005-10-01 [Environ. Toxicol. Chem. 24(10) , 2401-9, (2005)] |
|
Comparison of rat olfactory mucosal responses to carcinogeni...
2009-06-01 [Food Chem. Toxicol. 47(6) , 1051-7, (2009)] |
|
Pesticide retention in an experimental wetland treating non-...
2007-01-01 [Water Sci. Technol. 55(3) , 37-44, (2007)] |