| Structure | Name/CAS No. | Articles |
|---|---|---|
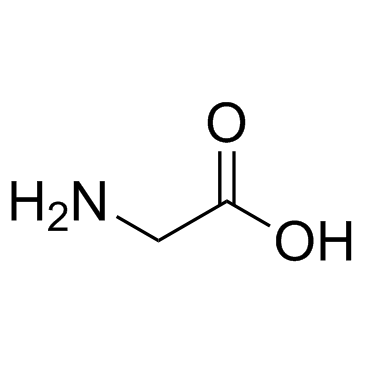 |
Glycine
CAS:56-40-6 |
|
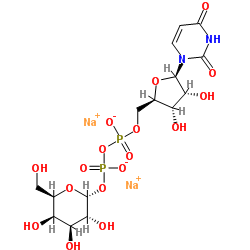 |
udp-alpha-d-galactose disodium salt
CAS:137868-52-1 |