| Structure | Name/CAS No. | Articles |
|---|---|---|
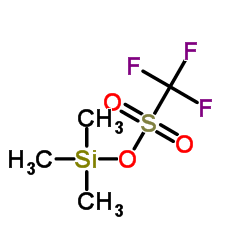 |
Trimethylsilyl trifluoromethanesulfonate
CAS:27607-77-8 |
|
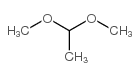 |
Dimethyl Acetal
CAS:534-15-6 |