| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
HYDROFLUORIC ACID
CAS:7664-39-3 |
|
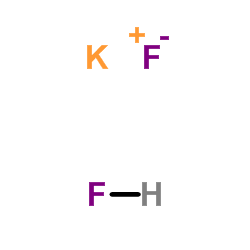 |
Potassium fluoride hydrofluoride (1:1:1)
CAS:7789-29-9 |