[Calcium carbide of different crystal formation synthesized by calcium carbide residue].
Zhong-yuan Lu, Ming Kang, Cai-rong Jiang, Ming-jing Tu
Index: Huan Jing Ke Xue 27(4) , 775-8, (2006)
Full Text: HTML
Abstract
To recycle calcium carbide residue effectively, calcium carbide of different crystal form, including global aragonite, calcite and acicular calcium carbide was synthesized. Both the influence of pretreatment in the purity of calcium carbide, and the influence of temperatures of carbonization reaction, release velocity of carbon dioxide in the apparition of calcium carbide of different crystal form were studied with DTA-TG and SEM. The result shows that calcium carbide residue can take place chemistry reaction with ammonia chlorinate straight. Under the condition that pH was above 7, the purity of calcium carbide was above 97%, and the whiteness was above 98. Once provided the different temperatures of carbonization reaction and the proper release velocity of carbon dioxide, global aragonite, calcite and acicular calcium carbide were obtained.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
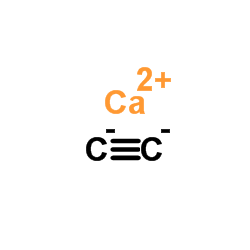 |
Calcium carbide
CAS:75-20-7 |
C2Ca |
|
Slow-release of methanogenic inhibitors derived from encapsu...
2011-11-01 [Waste Manag Res 29(11) , 1197-204, (2011)] |
|
[Severe ocular burns by calcium carbide in a speleologist: a...
2002-03-01 [J. Fr. Ophtalmol. 25(3) , 308-11, (2002)] |
|
Pressure-induced superconductivity in CaC2.
2013-06-04 [Proc. Natl. Acad. Sci. U. S. A. 110(23) , 9289-94, (2013)] |
|
Determination of total body water by a simple and rapid mass...
1996-01-01 [J. Mass Spectrom. 31(1) , 108-11, (1996)] |
|
[Nasal septum perforation caused by calcium carbide dust].
1989-01-01 [Z. Arztl. Fortbild. (Jena.) 83(24) , 1267-9, (1989)] |