Divergent mechanisms of suicide inactivation for ethanolamine ammonia-lyase.
Gregory M Sandala, David M Smith, Leo Radom
Index: J. Am. Chem. Soc. 127(24) , 8856-64, (2005)
Full Text: HTML
Abstract
Ab initio molecular orbital calculations have been used to study the mechanism of suicide inactivation of ethanolamine ammonia-lyase induced by three different substrate analogues. Analysis of the normal catalytic mechanism with 2-aminoethanol (ethanolamine) as substrate predicts that both the hydrogen-abstraction and hydrogen-reabstraction steps involving the B(12)-cofactor are likely to be exothermic. On the other hand, the proposed inactivation mechanism for the first substrate analogue, glycolaldehyde, leads to a highly stabilized radical that results in a very endothermic (by ca. 90 kJ mol(-)(1)) hydrogen-reabstraction step, which is thought to halt the normal function of the enzyme. Curiously, the energy requirements for a catalytically imposed mechanism in the case of the second substrate analogue, 2-hydroxyethylhydrazine (HEH), parallel those for the catalytic substrate, despite the fact that HEH is found to inactivate EAL experimentally. However, further analysis reveals the presence of a lower energy pathway for HEH that leads to the formation of the highly stabilized hydrazinium radical cation. In a manner similar to when glycolaldehyde is the substrate analogue, this results in an endothermicity for the hydrogen-reabstraction step that is prohibitively large. In contrast to these related inactivation mechanisms, the third substrate analogue, 2-aminoacetaldehyde, apparently accomplishes the inactivation of EAL in an entirely different manner. A pathway for the experimentally observed formation of acetic acid and ammonium cation has been identified and appears catalytic in the sense that 5'-deoxyadenosyl radical is regenerated. However, mechanisms to account for the subsequent formation of 4',5'-anhydroadenosine and degradation of the corrinoid ring of the cofactor have not been elucidated.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
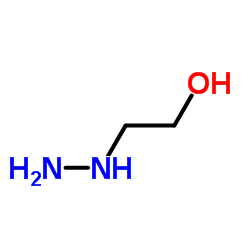 |
2-Hydrazinoethanol
CAS:109-84-2 |
C2H8N2O |
|
X-ray Single Crystal Structure, DFT Calculations and Biologi...
2016-01-01 [Molecules 21(8) , doi:10.3390/molecules21081020, (2016)] |
|
Use of pig hepatocytes to study the inhibition of monoamine ...
1991-03-01 [Food Chem. Toxicol. 29(3) , 185-91, (1991)] |
|
Electron-microscopic cytochemical localization of diamine an...
1991-02-01 [Planta 183(3) , 443-50, (1991)] |
|
2-hydroxyethylhydrazine as a potent inhibitor of phospholipi...
1983-04-13 [Biochim. Biophys. Acta 751(2) , 201-9, (1983)] |
|
Light promotes the synthesis of lignin through the productio...
2005-12-01 [J. Plant Physiol. 162(12) , 1297-303, (2005)] |