| Structure | Name/CAS No. | Articles |
|---|---|---|
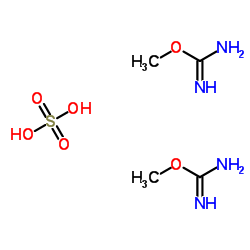 |
O-Methylisourea hemisulfate
CAS:52328-05-9 |
|
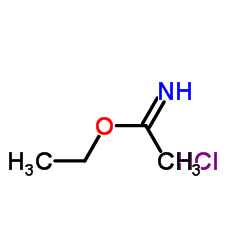 |
Ethyl acetimidate hydrochloride
CAS:2208-07-3 |
|
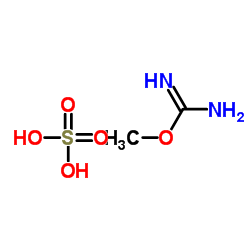 |
Methyl carbamimidate sulfate
CAS:29427-58-5 |