| Structure | Name/CAS No. | Articles |
|---|---|---|
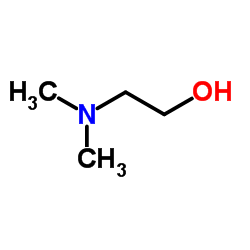 |
2-(Dimethylamino)ethanol
CAS:108-01-0 |
|
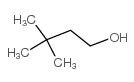 |
3,3-DIMETHYL-1-BUTANOL
CAS:624-95-3 |
|
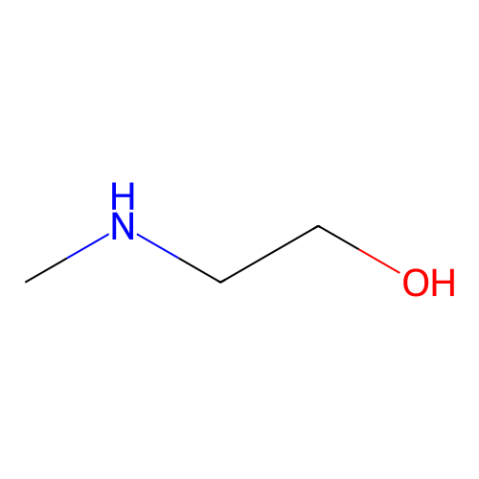 |
2-Methylaminoethanol
CAS:109-83-1 |