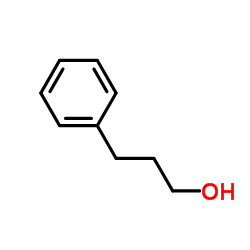
|
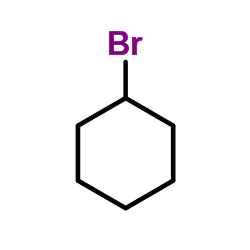
~83% |
|
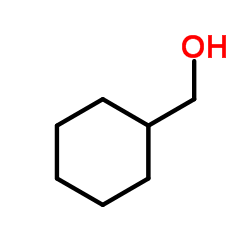
~89% |
|
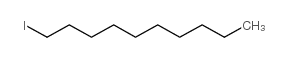
~56% |
|
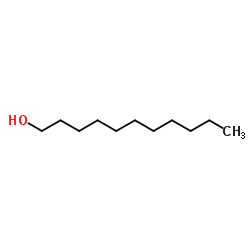
~24% |
|
~77% |
|
~41% |
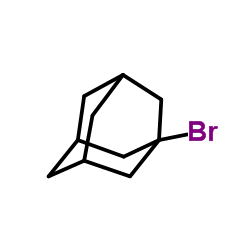


![Tricyclo[3.3.1.13,7]decane,1-iodo Structure](https://image.chemsrc.com/caspic/081/768-93-4.png)