| Structure | Name/CAS No. | Articles |
|---|---|---|
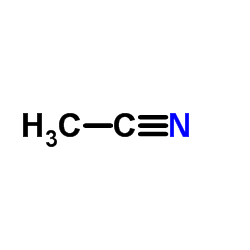 |
Acetonitrile
CAS:75-05-8 |
|
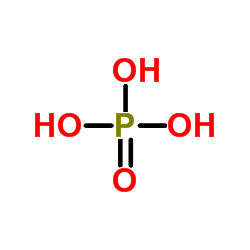 |
Phosphoric acid
CAS:7664-38-2 |
|
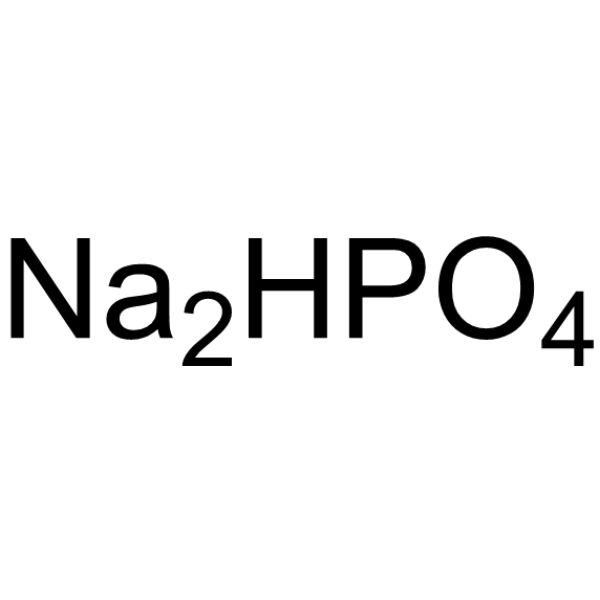 |
Disodium hydrogenorthophosphate
CAS:7558-79-4 |
|
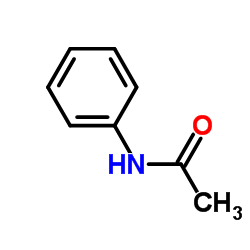 |
Acetanilide
CAS:103-84-4 |
|
 |
HR 111V sulfate
CAS:118443-89-3 |