| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
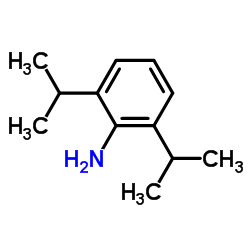 |
2,6-Diisopropylaniline
CAS:24544-04-5 |
|
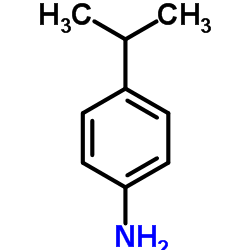 |
4-Isopropylaniline
CAS:99-88-7 |
|
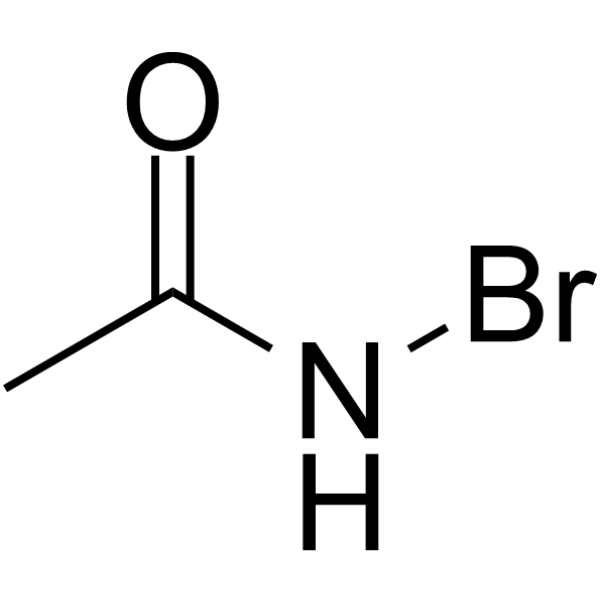 |
N-Bromoacetamide
CAS:79-15-2 |
|
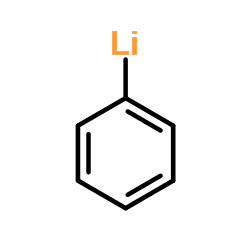 |
Phenyllithium
CAS:591-51-5 |
|
 |
Stannous chloride dihydrate
CAS:10025-69-1 |
|
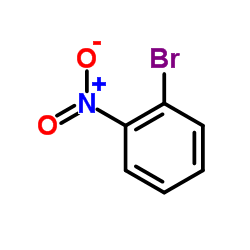 |
1-Bromo-2-nitrobenzene
CAS:577-19-5 |