| Structure | Name/CAS No. | Articles |
|---|---|---|
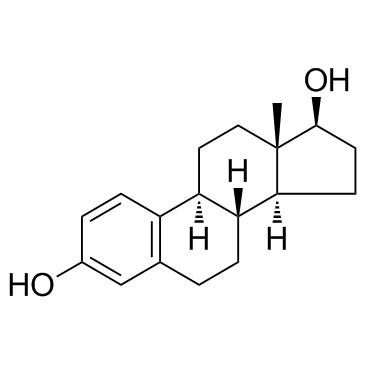 |
beta-Estradiol
CAS:50-28-2 |
|
 |
Phenol
CAS:108-95-2 |
|
 |
p-Cresol
CAS:106-44-5 |
|
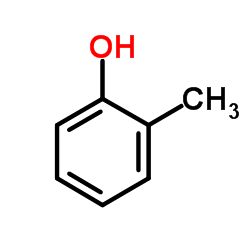 |
o-cresol
CAS:95-48-7 |
|
 |
Peratox
CAS:87-86-5 |
|
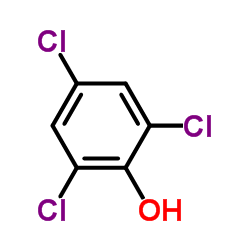 |
2,4,6-Trichlorophenol
CAS:88-06-2 |
|
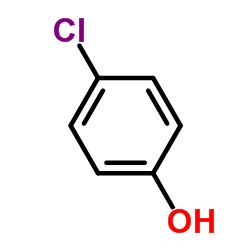 |
4-Chlorophenol
CAS:106-48-9 |
|
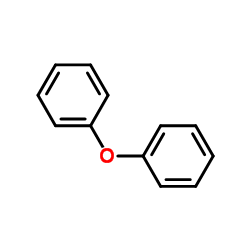 |
Diphenyl oxide
CAS:101-84-8 |
|
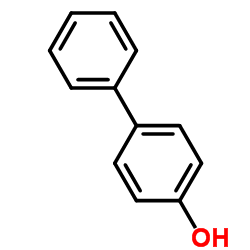 |
4-Biphenylol
CAS:92-69-3 |
|
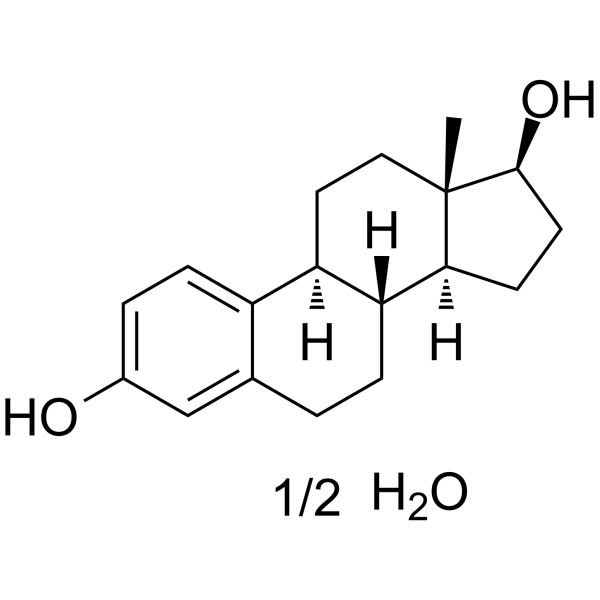 |
ESTRADIOL HEMIHYDRATE
CAS:35380-71-3 |