| Structure | Name/CAS No. | Articles |
|---|---|---|
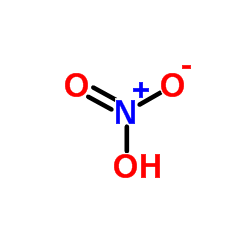 |
nitric acid
CAS:7697-37-2 |
|
 |
L-cysteine
CAS:52-90-4 |
|
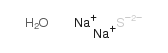 |
Sodium sulphide hydrate
CAS:27610-45-3 |
|
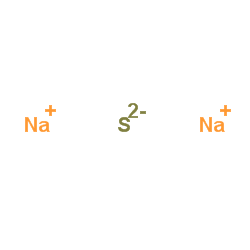 |
Sodium sulfide
CAS:1313-82-2 |