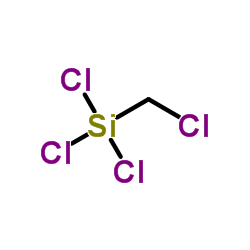
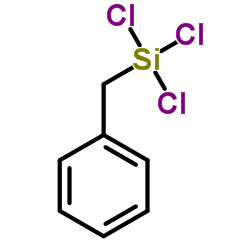
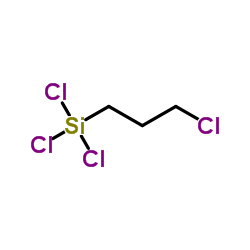
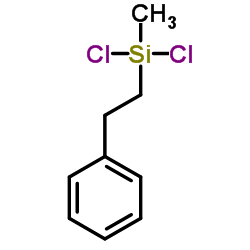
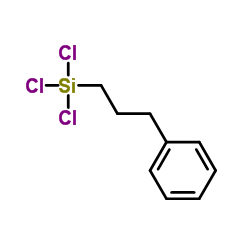
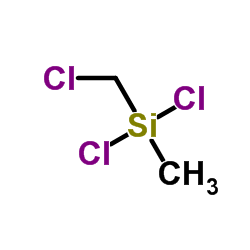
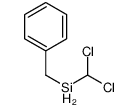
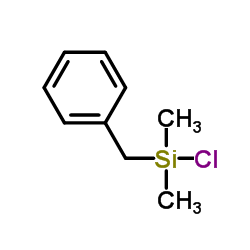
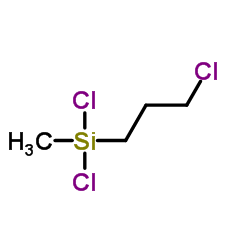
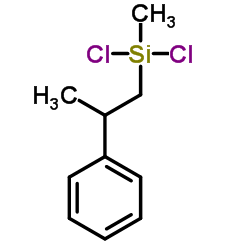
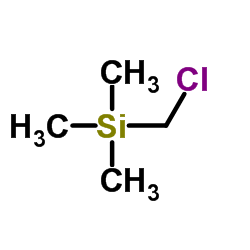
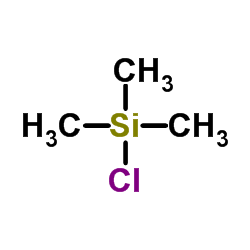
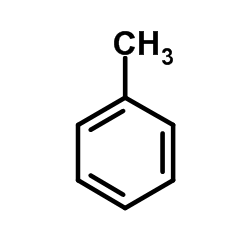
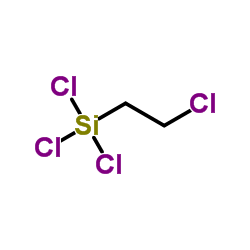
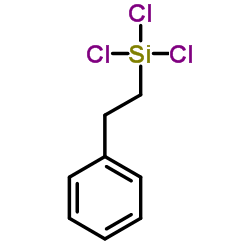
|
~31% |
|
~89% |
|
~71% |
|
~17%
Detail
|
|
~80% |
|
~% |
|
~13% |
|
~89% |