| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Lithium fluoride
CAS:7789-24-4 |
|
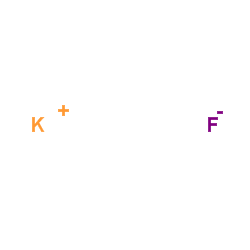 |
Potassium fluoride
CAS:7789-23-3 |
|
 |
Lithium-6Li fluoride
CAS:14885-65-5 |