Efficient access to cyclic ureas via Pd-catalyzed cyclization.
Mark McLaughlin, Michael Palucki, Ian W Davies
Index: Org. Lett. 8 , 3311, (2006)
Full Text: HTML
Abstract
[Structure: see text] An efficient regioselective method for the preparation of structurally diverse imidazopyridinones and benzoimidazolones starting from readily available and economical starting materials is described. High-yielding reductive alkylation of electron-deficient o-haloarylamines followed by treatment with inexpensive N-chlorosulfonyl isocyanate afforded primary ureas in good overall yields. A Pd-catalyzed urea cyclization reaction furnished imidazopyridinones and benzoimidazolones in excellent yields. Overall, the developed chemistry provides rapid access to pharmaceutically important heterocyclic compounds with high efficiency.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
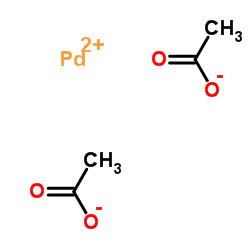 |
Palladium diacetate
CAS:3375-31-3 |
C4H6O4Pd |
|
A sulfoxide-promoted, catalytic method for the regioselectiv...
2004-02-11 [J. Am. Chem. Soc. 126 , 1346, (2004)] |
|
Palladium(II) acetate in pyridine as an effective catalyst f...
2005-01-21 [J. Org. Chem. 70 , 696, (2005)] |
|
Preparation of the membrane-permeant biarsenicals FlAsH-EDT2...
2008-01-01 [Nat. Protoc. 3(9) , 1527-34, (2008)] |
|
Synthesis of chiral heterocycles by ligand-controlled regiod...
2014-01-01 [Nat. Commun. 5 , 5474, (2014)] |
|
Badone, D.; Guzzi, U.
[Tetrahedron Lett. 34 , 3603, (1993)] |