| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
L-Isoleucine
CAS:73-32-5 |
|
 |
ec 1.1.1.47
CAS:9028-53-9 |
|
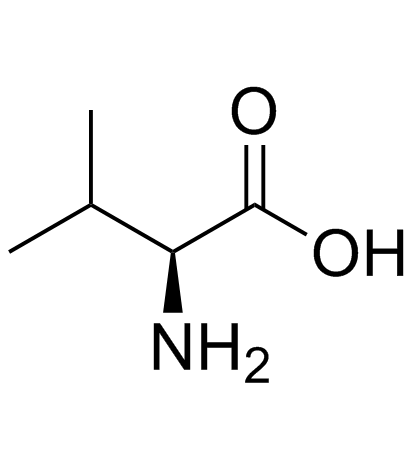 |
L-Valine
CAS:72-18-4 |