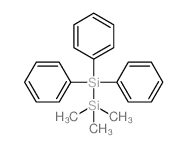
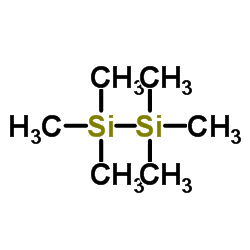
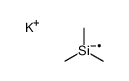
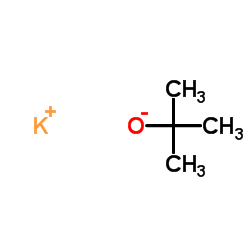
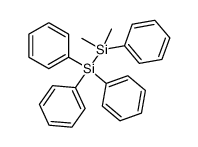
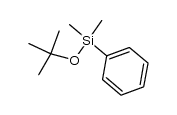
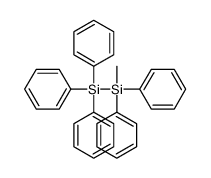
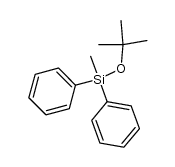
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~71% |
|
~70% |
|
~95% |
|
~90% |
|
~40% |
|
~30% |
|
~97% |
|
~91% |
|
~39% |
|
~30% |