| Structure | Name/CAS No. | Articles |
|---|---|---|
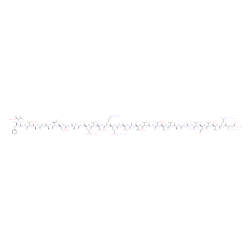 |
α-Synuclein (61-95) (human) trifluoroacetate salt
CAS:154040-19-4 |
|
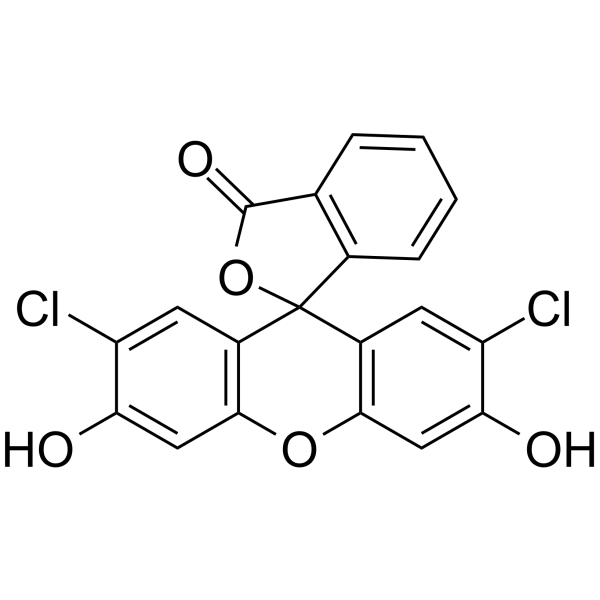 |
dichlorofluorescein
CAS:76-54-0 |