| Structure | Name/CAS No. | Articles |
|---|---|---|
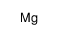 |
Magnesium
CAS:7439-95-4 |
|
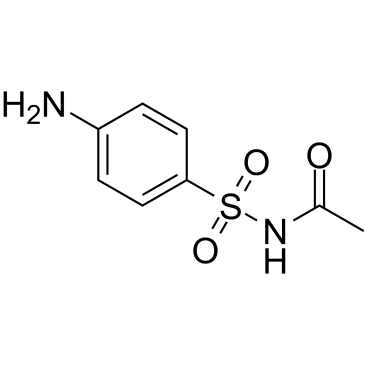 |
sulfacetamide
CAS:144-80-9 |
|
 |
Argipressin
CAS:113-79-1 |
|
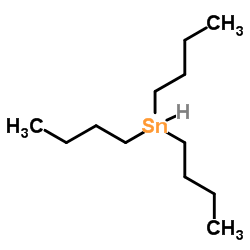 |
Tributyltin hydride
CAS:688-73-3 |
|
 |
GHRP-6 Acetate
CAS:87616-84-0 |