Halofantrine and chloroquine inhibit CYP2D6 activity in healthy Zambians.
O O Simooya, G Sijumbil, M S Lennard, G T Tucker
Index: Br. J. Clin. Pharmacol. 45(3) , 315-7, (1998)
Full Text: HTML
Abstract
To determine the effect of therapeutic loading doses of halofantrine and chloroquine on CYP2D6 activity in healthy black Zambians.Twenty healthy black male Zambians were phenotyped for CYP2D6 activity by measuring the debrisoquine/4-hydroxydebrisoquine ratio in a 0-8 h urine sample after a 10 mg oral dose of debrisoquine hemi-sulphate. The subjects (all 'extensive metabolizer' phenotype with respect to CYP2D6) were randomized into two groups of 10, and 24 h later one group received 1500 mg halofantrine hydrochloride and the other group 1500 mg chloroquine phosphate both orally in divided doses. All subjects were given further 10 mg doses of debrisoquine at 2 h, 1 week and 2 weeks after the last dose of the antimalarial drug, and phenotyped as described above.The median debrisoquine/4-hydroxydebrisoquine 0-8 h urinary ratio was increased by halofantrine (1.39 to 6.05; P<0.01; 95% confidence intervals 4.00-11.7) and chloroquine (1.96 to 3.91; P<0.01; 95% confidence intervals 1.34-2.66) when debrisoquine was given 2 h after treatment. The decrease in CYP2D6 activity remained statistically significant for 1 week after both drugs. Halofantrine was a significantly more potent inhibitor of CYP2D6 than chloroquine (P=0.037). Phenocopying occurred in two subjects taking halofantrine and one taking chloroquine (i.e. the debrisoquine/4-hydroxydebrisoquine ratios became consistent with the poor metabolizer phenotype).Given in therapeutic loading doses, both halofantrine and chloroquine caused significant inhibition of CYP2D6 activity in healthy black Zambians. With respect to halofantrine, this finding reinforces the recommendation that its combination with other drugs known to prolong the QT interval should be avoided, especially those that are metabolized significantly by CYP2D6.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
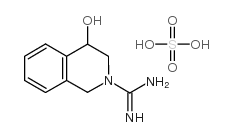 |
rac 4-Hydroxydebrisoquine Hemisulfate
CAS:62580-84-1 |
C10H15N3O5S |
|
Microsomal formation of nitric oxide and cyanamides from non...
1999-08-01 [Biochem. Pharmacol. 58(3) , 439-45, (1999)] |
|
Determination of debrisoquine and 4-hydroxydebrisoquine by h...
2005-01-01 [Clin. Chem. Lab Med. 43(3) , 275-9, (2005)] |
|
Enantioselectivity in the steady-state pharmacokinetics of m...
1999-01-01 [Chirality 11(7) , 591-7, (1999)] |
|
Debrisoquine hydroxylation in Parkinson's disease.
1992-08-01 [Acta Neurol. Scand 86(2) , 159-64, (1992)] |
|
Determination of debrisoquine and 4-hydroxydebrisoquine in u...
1993-08-01 [J. Pharm. Biomed. Anal. 11(8) , 745-9, (1993)] |