N-acetylator variability in Down's syndrome: characterization with caffeine.
M E Morris, J C Griener, M E Msall
Index: Clin. Pharmacol. Ther. 46(3) , 359-66, (1989)
Full Text: HTML
Abstract
Little is known regarding the biotransformation of drugs in Down's syndrome. In particular, there are no published studies that examine metabolic pathways such as N-acetylation, which can exhibit genetically-determined variability. The objective of the present investigation was to compare the acetylator phenotypes of white subjects with Down's syndrome with age-matched control subjects, with use of caffeine as the pharmacologic probe. After the ingestion of caffeine-containing beverages, spot urine collections were obtained at 2 and 4 hours in 22 subjects with Down's syndrome and in 22 control subjects (age range of 4 to 49 years). The urinary excretion ratios of 5-acetylamino-6-amino-3-methyluracil (AAMU) to 1-methylxanthine (1X) determined in these 2-hour and 4-hour samples were highly correlated (r = 0.82; p less than 0.001). In addition, more extensive urinary excretion studies performed for an 8-hour period in three subjects with Down's syndrome demonstrated that the coefficient of variability for the ratio of AAMU/1X ranged from 10.1% to 14.2%, which is similar to the reproducibility previously reported for control subjects. A trimodal distribution of acetylator phenotypes was observed, with no differences in average or frequency distribution of ratio values between the subjects with Down's syndrome and the control subjects. This study demonstrates that polymorphic N-acetylator status, as assessed by caffeine metabolism, is similar in subjects with Down's syndrome and in control subjects.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
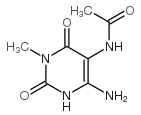 |
N-(6-amino-3-methyl-2,4-dioxo-1H-pyrimidin-5-yl)acetamide
CAS:19893-78-8 |
C7H10N4O3 |
|
Caffeine as a metabolic probe: validation of its use for ace...
1991-06-01 [Clin. Pharmacol. Ther. 49(6) , 648-57, (1991)] |
|
An alternative test for acetylator phenotyping with caffeine...
1987-11-01 [Clin. Pharmacol. Ther. 42(5) , 509-13, (1987)] |
|
Extractionless method for the simultaneous high-performance ...
2001-05-05 [J. Chromatogr. B. Biomed. Sci. Appl. 755(1-2) , 73-84, (2001)] |
|
A competitive enzyme linked immunosorbent assay for the dete...
1995-08-01 [J. Pharm. Biomed. Anal. 13(9) , 1079-86, (1995)] |
|
Human N-acetylation genotype determination with urinary caff...
1990-04-01 [Clin. Pharmacol. Ther. 47(4) , 470-7, (1990)] |