Phosphine-initiated general base catalysis: facile access to benzannulated 1,3-diheteroatom five-membered rings via double-Michael reactions of allenes.
Judy Szeto, Vardhineedi Sriramurthy, Ohyun Kwon
Index: Org. Lett. 13(20) , 5420-3, (2011)
Full Text: HTML
Abstract
General base-catalyzed double-Michael reactions of allenes with various dinucleophiles are described. The reactions are facilitated most efficiently by a catalytic amount of trimethylphosphine, affording six types of C2-functionalized benzannulated five-membered heterocycles: benzimidazolines, benzoxazolines, benzothiazolines, 1,3-benzodioxoles, 1,3-benzoxathioles, and 1,3-benzodithioles. This atom-economical reaction is operationally simple and provides the product heterocycles in good to excellent yields. Careful mechanistic studies unveiled the phosphine-triggered general base catalysis pathway. Furthermore, the double-Michael reaction can serve as an alternative method for the selective monoketalization of β-diketones.© 2011 American Chemical Society
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
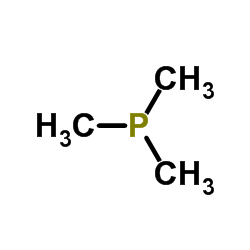 |
Trimethylphosphine
CAS:594-09-2 |
C3H9P |
|
Change of surface structure upon foam film formation.
2015-03-16 [ChemPhysChem 16(4) , 733-8, (2015)] |
|
Molybdenum complex with bulky chelates as a functional model...
2014-12-01 [Inorg. Chem. 53(23) , 12416-27, (2014)] |
|
Trimethylphosphine binding to horse-heart and sperm-whale my...
1993-06-01 [Eur. J. Biochem. 214(2) , 405-14, (1993)] |
|
31P-NMR investigation of trimethylphosphine binding to [alph...
1990-08-31 [Biophys. Chem. 37(1-3) , 407-11, (1990)] |
|
Synthesis and evaluation of technetium-99m monocationic mixe...
1992-08-01 [Int. J. Rad. Appl. Instrum. B 19(6) , 679-84, (1992)] |