Orthoacylimines: a new class of chiral auxiliaries for nucleophilic addition of organolithium reagents to imines.
Alessandro A Boezio, Geoffrey Solberghe, Caroline Lauzon, André B Charette
Index: J. Org. Chem. 68 , 3241, (2003)
Full Text: HTML
Abstract
A new class of orthoacylimine-derived chiral auxiliaries has been synthesized and tested in the nucleophilic addition of organolithium reagents to imines. The precursors can be prepared by an aza-Wittig reaction between the corresponding orthoacyl azide and a variety of aldehydes in the presence of trialkylphosphines. The nucleophilic addition of organolithium reagents led to the addition products in good yields and with good to excellent diastereoselectivities (from 85:15 to 99:1). The chiral, nonracemic secondary amines could be readily obtained under mild hydrolytic condition. Furthermore, the chiral auxiliary can be recovered in quantitative yield and reconverted to the starting orthoacyl azide precursor. This method was applied to the synthesis of (S)-t-leucine.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
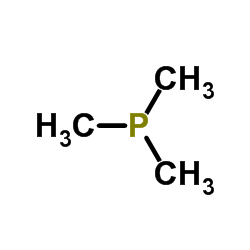 |
Trimethylphosphine
CAS:594-09-2 |
C3H9P |
|
Change of surface structure upon foam film formation.
2015-03-16 [ChemPhysChem 16(4) , 733-8, (2015)] |
|
Molybdenum complex with bulky chelates as a functional model...
2014-12-01 [Inorg. Chem. 53(23) , 12416-27, (2014)] |
|
Trimethylphosphine binding to horse-heart and sperm-whale my...
1993-06-01 [Eur. J. Biochem. 214(2) , 405-14, (1993)] |
|
31P-NMR investigation of trimethylphosphine binding to [alph...
1990-08-31 [Biophys. Chem. 37(1-3) , 407-11, (1990)] |
|
Synthesis and evaluation of technetium-99m monocationic mixe...
1992-08-01 [Int. J. Rad. Appl. Instrum. B 19(6) , 679-84, (1992)] |