Function-oriented biosynthesis of beta-lactone proteasome inhibitors in Salinispora tropica.
Markus Nett, Tobias A M Gulder, Andrew J Kale, Chambers C Hughes, Bradley S Moore
Index: J. Med. Chem. 52(19) , 6163-7, (2009)
Full Text: HTML
Abstract
The natural proteasome inhibitor salinosporamide A from the marine bacterium Salinispora tropica is a promising drug candidate for the treatment of multiple myeloma and mantle cell lymphoma. Using a comprehensive approach that combined chemical synthesis with metabolic engineering, we generated a series of salinosporamide analogues with altered proteasome binding affinity. One of the engineered compounds is equipotent to salinosporamide A in inhibition of the chymotrypsin-like activity of the proteasome yet exhibits superior activity in the cell-based HCT-116 assay.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
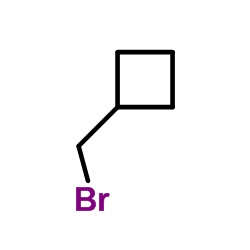 |
Cyclobutylmethyl bromide
CAS:17247-58-4 |
C5H9Br |
|
Synthesis and opioid receptor binding affinities of 2-substi...
2010-01-14 [J. Med. Chem. 53(1) , 402-18, (2010)] |
|
Replacement surgery with unnatural amino acids in the lock-a...
2006-09-01 [Chem. Biol. 13(9) , 929-36, (2006)] |
|
10-Ketomorphinan and 3-substituted-3-desoxymorphinan analogu...
2004-01-01 [J. Med. Chem. 47 , 165, (2004)] |
|
Photochemical synthesis and antimicrobial studies of new chr...
[Arabian Journal of Chemistry , (2013)] |
|
Cyclopropylmethyl-and cyclobutylmethyllithium by an arene-ca...
[Tetrahedron 66(16) , 2928-35, (2010)] |