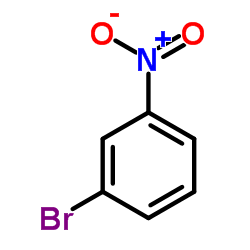
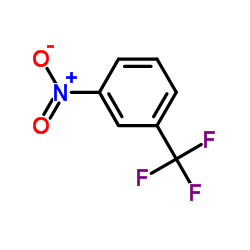
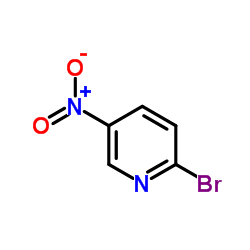
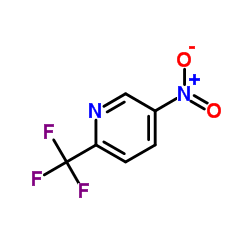
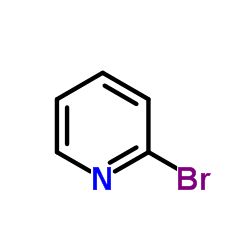
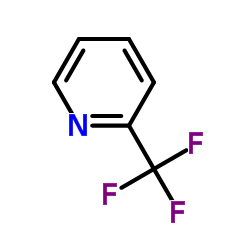
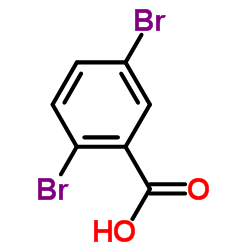
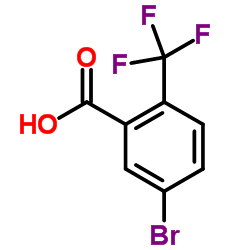
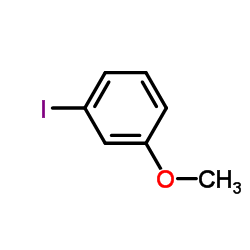
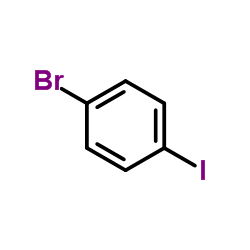
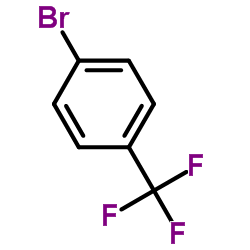
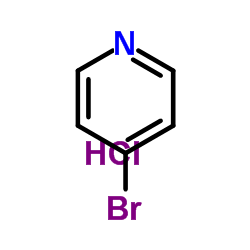
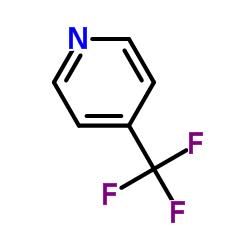
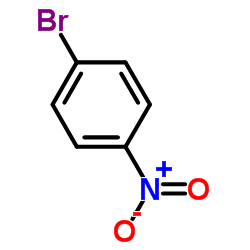
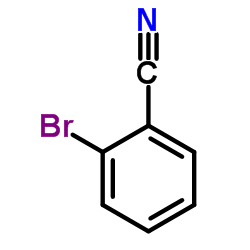
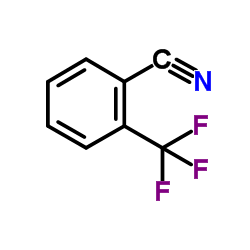
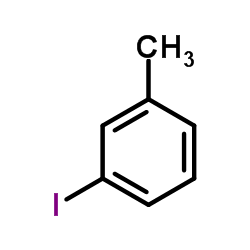
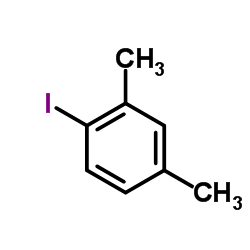
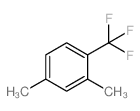
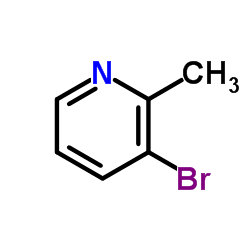
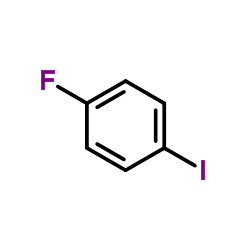
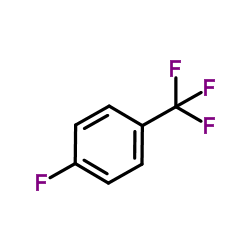
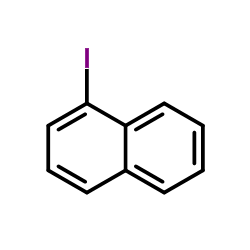
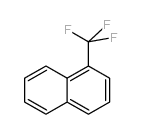
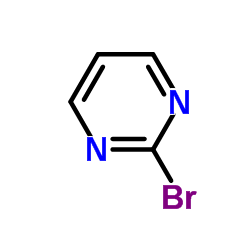
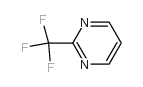
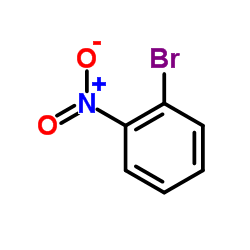
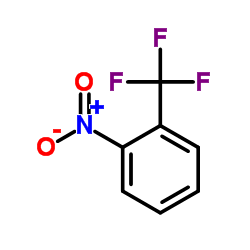
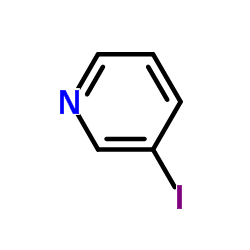
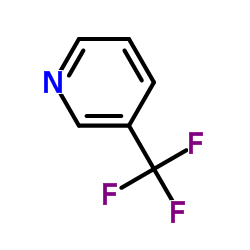
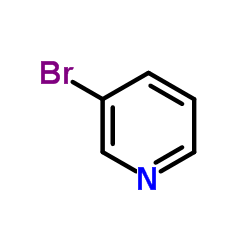
|
~99% |
|
~94% |
|
~56% |
|
~74% |
|
~33% |
|
~77% |
|
~88% |
|
~91% |
|
~% |
|
~30% |
|
~91% |
|
~77% |
|
~22% |
|
~89% |
|
~87% |
|
~82% |
|
~94% |
|
~96% |
|
~75% |
|
~60% |
|
~29% |
|
~99% |
|
~99% |
|
~20% |
|
~84% |
|
~97% |
|
~95% |
|
~88% |
|
~87% |
|
~8% |
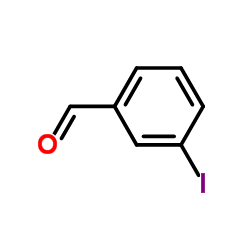


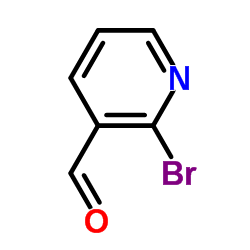
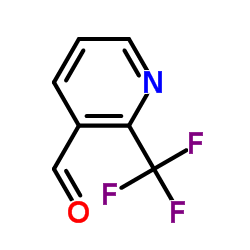
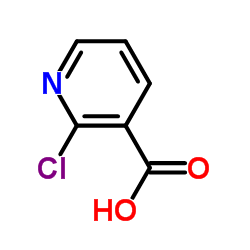
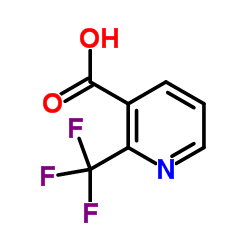

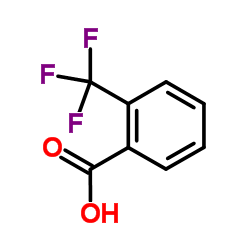
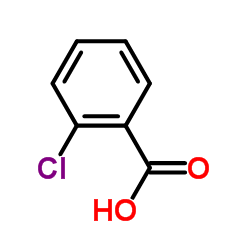
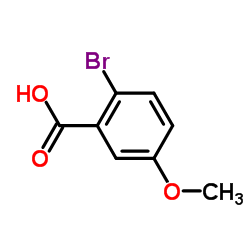
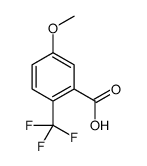
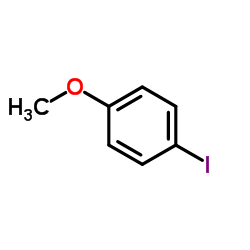
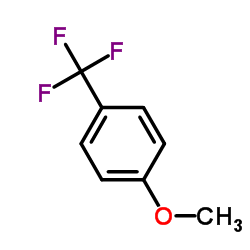

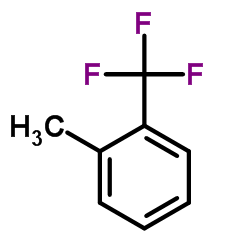
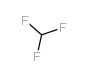

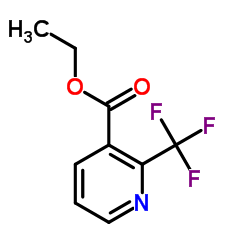
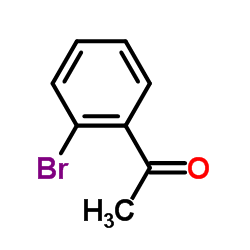
![1-[2-(Trifluoromethyl)phenyl]ethanone Structure](https://image.chemsrc.com/caspic/206/17408-14-9.png)