| Structure | Name/CAS No. | Articles |
|---|---|---|
![8-chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepin-4-ol Structure](https://image.chemsrc.com/caspic/343/59468-85-8.png) |
8-chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepin-4-ol
CAS:59468-85-8 |
|
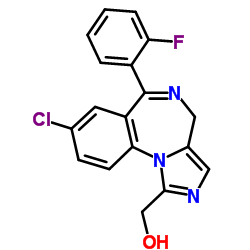 |
1'-Hydroxymidazolam
CAS:59468-90-5 |