| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Potassium bromide
CAS:7758-02-3 |
|
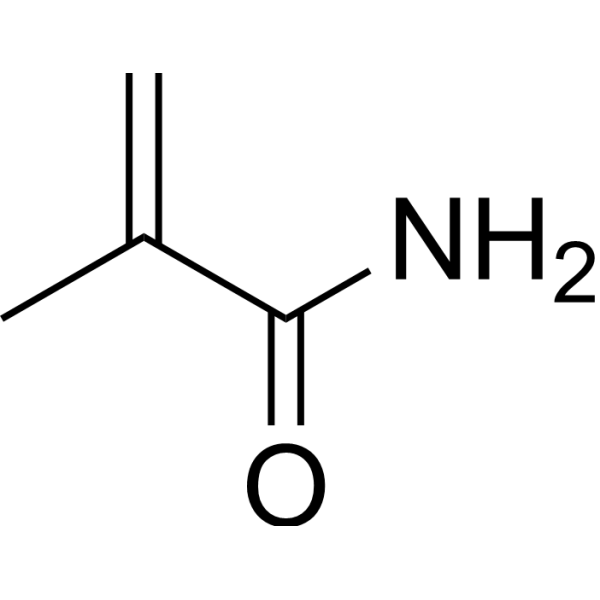 |
Methacrylamide
CAS:79-39-0 |
|
 |
Physostigmine
CAS:57-47-6 |