Enzymatic preparation of 5-hydroxy-L-proline, N-Cbz-5-hydroxy-L-proline, and N-Boc-5-hydroxy-L-proline from (α-N-protected)-L-ornithine using a transaminase or an amine oxidase.
R L Hanson, R M Johnston, S L Goldberg, W L Parker, R N Patel, R.L. Hanson, R.M. Johnston, S.L. Goldberg, W.L. Parker, R.N. Patel
Index: Enzyme Microb. Technol. 48(6-7) , 445-53, (2011)
Full Text: HTML
Abstract
N-Cbz-4,5-dehydro-L-prolineamide or N-Boc-4,5-dehydro-L-prolineamide are alternative key intermediates for the synthesis of saxagliptin, a dipeptidyl peptidase IV (DPP4) inhibitor recently approved for treatment of type 2 diabetes mellitus. An efficient biocatalytic method was developed for conversion of L-ornithine, N-α-benzyloxycarbonyl (Cbz)-L-ornthine, and N-α-tert-butoxycarbonyl (Boc)-L-ornithine to 5-hydroxy-L-proline, N-Cbz-5-hydroxy-L-proline, and N-Boc-5-hydroxy-L-proline, respectively. Rec. Escherichia coli expressing lysine-ε-aminotransferase and rec Pichia pastoris expressing L-ornithine oxidase were used for these conversions. N-Cbz-5-hydroxy-L-proline, and N-Boc-5-hydroxy-L-proline were chemically converted to key intermediates N-Cbz-4,5-dehydro-L-prolineamide and N-Boc-4,5-dehydro-L-prolineamide, respectively.Copyright © 2011 Elsevier Inc. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
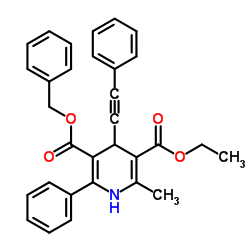 |
L-Amino acid oxidase
CAS:9000-89-9 |
C31H27NO4 |
|
The status, quality, and expansion of the NIH full-length cD...
2004-10-01 [Genome Res. 14 , 2121-7, (2004)] |
|
Mechanisms of action of escapin, a bactericidal agent in the...
2012-04-01 [Antimicrob. Agents Chemother. 56(4) , 1725-34, (2012)] |
|
Advances in non-snake venom L-amino acid oxidase.
2012-05-01 [Appl. Biochem. Biotechnol. 167(1) , 1-13, (2012)] |
|
Phylogenetic analysis supports horizontal gene transfer of L...
2012-07-01 [Infect. Genet. Evol. 12(5) , 1005-9, (2012)] |
|
L-amino acid oxidase-induced apoptosis in filamentous Botryt...
2012-01-01 [Anal. Biochem. 420(1) , 93-5, (2012)] |