Potentiation of halomethane hepatotoxicity: chlordecone and carbon tetrachloride.
H M Mehendale
Index: Fundam. Appl. Toxicol. 4(3 Pt 1) , 295-308, (1984)
Full Text: HTML
Abstract
The propensity for chlordecone to potentiate hepatotoxicity of CCl4 and some related analogs (CHCl3 and CBrCl3) has been well established. The interaction results in potentiation of halomethane hepatotoxicity at incredibly small, nontoxic levels of chlordecone and these halomethanes. Biological effects of such interactions include extensive hepatotoxicity characterized by total hepatic failure as determined by histopathological alterations, hepatic dysfunction, elevation of serum enzymes, and perturbation of related biochemical parameters. The chlordecone + CCl4 interaction occurs in animals of both sexes and is characterized by profoundly potentiated lethality. Close structural analogs of chlordecone such as mirex and photomirex do not share the propensity of chlordecone to potentiate halomethane toxicity. The mechanism of this potentiation of halomethane hepatotoxicity has eluded several investigations. Induction of microsomal P-450 by chlordecone and greater lipid peroxidation appear inadequate to explain the powerful potentiation of toxicity. Time-course studies in which liver tissue was examined 1 to 36 hr after CCl4 administration to chlordecone treated animals revealed possible mechanisms. It appears that a greater bioactivation of CCl4 in chlordecone treated animals resulted in an initial potentiation of toxic events in the liver cells. While animals receiving a normally nontoxic dose of CCl4 alone demonstrate repair and renovation of liver tissue as revealed by greatly increased mitotic index after 12 hours, such a renovation process is totally suppressed in animals exposed to chlordecone. Thus, a combination of initial greater injury along with prevention of hepatocellular repair and renovation appears to play a key role in the potentiation of liver injury. The greater initial liver injury is consistent with enhanced metabolism and bioactivation of CCl4 in chlordecone exposed animals. The mechanism of suppressed cellular mitosis is less clearly understood. Recent studies have revealed a greater perturbation of intracellular Ca2+ homeostasis in chlordecone treated animals and this observation is consistent with continued destruction and cellular damage. These observations point to more than a single factor involved as underlying mechanisms and two such mechanisms are consistent with the available information. First, chlordecone induction of a specific form of P-450 capable of greater bioactivation of CCl4. Second, chlordecone sensitization of hepatocellular plasma membrane for excessive accumulation of intracellular Ca2+.(ABSTRACT TRUNCATED AT 400 WORDS)
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
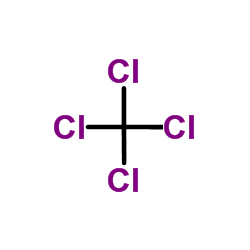 |
Carbon tetrachloride
CAS:56-23-5 |
CCl4 | |
![1,3,4-Metheno-2H-cyclobuta[cd]pentalen-2-one,1,1a,3,3a,4,5,5,5a,5b,6-decachlorooctahydro Structure](https://image.chemsrc.com/caspic/291/143-50-0.png) |
1,3,4-Metheno-2H-cyclobuta[cd]pentalen-2-one,1,1a,3,3a,4,5,5,5a,5b,6-decachlorooctahydro
CAS:143-50-0 |
C10Cl10O |
|
Cranberry flavonoids prevent toxic rat liver mitochondrial d...
2015-06-01 [Cell Biochem. Funct. 33 , 202-10, (2015)] |
|
Synthesis and Properties of a Novel Pyridineoxazoline Contai...
2015-08-01 [Chirality 27 , 523-31, (2015)] |
|
Optimized Dispersive Liquid-Liquid Microextraction Method an...
2015-01-01 [J. AOAC Int. 98 , 962-70, (2015)] |
|
Convenient QSAR model for predicting the complexation of str...
2009-01-01 [Bioorg. Med. Chem. 17 , 896-904, (2009)] |
|
The Japanese toxicogenomics project: application of toxicoge...
2010-02-01 [Mol. Nutr. Food. Res. 54 , 218-27, (2010)] |